Abich carries out efficacy testing on cosmetics, medical devices, raw materials, pharmaceutical products, OTCs, and other consumer products, in full compliance with current regulations.
Once the safety of a product is verified, efficacy studies confirm the claims.
Abich’s Cell Biology and Toxicology Laboratory performs in-vitro efficacy tests on specific cell lines and in-vitro reconstructed human tissue.
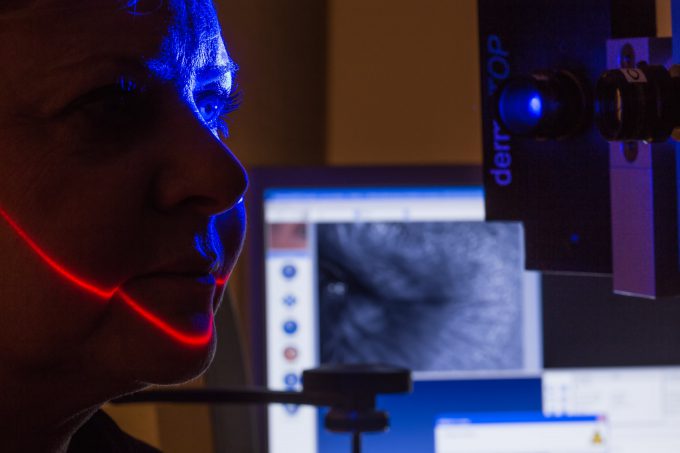
The clinical division of Abich laboratories performs efficacy tests on healthy volunteers under the supervision of dermatologists, allergologists, and other medical specialists.
For any type of product claiming different properties, Abich designs experimental protocols tailored to meet customers’ needs.
In-vitro efficacy testing offered by Abich:
- Antioxidant and antiradical activity testing: on skin cells (ROS analysis);
- Direct antioxidant activity testing;
- Skin anti-ageing and redensifying activity testing: mitogenic activity, stimulation of protein synthesis, stimulation of the protein synthesis by extracellular matrix (collagen, elastin, fibronectin, etc.);
- Anti-inflammatory activity testing: on skin cells and in-vitro reconstructed epithelia of different origin;
- Evaluation of pro-inflammatory potential: through the analysis of cytokines and growth factors (IL-1α, IL-1β, IL-8, GM-CSF, TNF-α);
- Cell proliferation testing: on skin cells;
- Melanogenesis modulation testing: increase/inhibition of melanin synthesis for depigmentation or pigmentation products;
- Percutaneous absorption testing: on skin reconstructed according to the OECD 428 method;
- Skin barrier testing : on reconstructed skin;
- Keratolytic effect assessment testing: on reconstructed skin;
- Wound healing activity testing: on endothelial cells and keratinocytes/fibroblasts;
- Immune response modulation testing: through the analysis of specific mediators (i.e., histamine, IL-1α, TNF-α) for anti-itch activity, inhibition of sensitization, immunostimulant activity;
- Evaluation of cellular senescence;
- Sebum normalization activity testing: lipase inhibition;
- Evaluation of 5-alpha-reductase inhibition;
- In-vitro moisturizing activity testing;
- In-vitro testing on sun protection products: UVA-PF in line with the ISO 24443: 2012/COLIPA 2011 standard, SPF UVA “Broad spectrum” in line with the FDA 2011 and AS/NZS 2604:2012 standard, UVA/UVB ratio in line with the Boots Star Rating 2008, blue light protection and IRA protection.
Clinical efficacy testing offered by Abich:
- Skin moisturizing testing: at short, medium, and long-term;
- Usability testing to evaluate the efficacy of cosmetic products under medical supervision;
- Anti-wrinkle activity testing: through reflection skin mapping;
- Skin elasticity enhancement testing: firming/toning;
- Skin barrier activity evaluation: (TEWL – Trans Epithelial Water Loss), Arm Wash Test;
- Tests for the reduction of eye bags and dark circles: through volumetric analysis and reflection skin mapping paired with cutaneous colorimetry;
- Depigmentation, lightening, and illuminating activity testing;
- Pigmentation activity testing: tan accelerating;
- Anti-cellulite activity testing;
- Sebum normalization or regulation testing;
- Comedogenetic testing;
- Antiperspirant activity testing: 24H, 48H (FDA protocol), and deodorant (Sniff Test);
- Lenitive activity testing: with chemically-induced irritation or UV radiation;
- Long-lasting testing: on face, eyelash, and lip make-up;
- Volumizing activity testing: on eyelash and lip make-up;
- Trichological product efficacy testing: anti-dandruff and hair loss prevention activity, hair vitality, hair breakage resistance, dye duration.