Abich performs safety testing on cosmetics, medical devices, raw materials, OTCs and other consumer products.
These products, once marketed, must be safe for human health when used in normal or reasonably foreseeable conditions. To this purpose, specific tests are carried out to safeguard both the consumers and the manufacturer.
Therefore, Abich performs preliminary in-vitro testing and later verifies the tolerability through in-vivo studies on healthy volunteers, in full compliance with current regulations.
Abich’s Cell Biology and Toxicology Laboratory performs on-vitro safety testing on specific cell lines and in-vitro reconstructed human tissue.
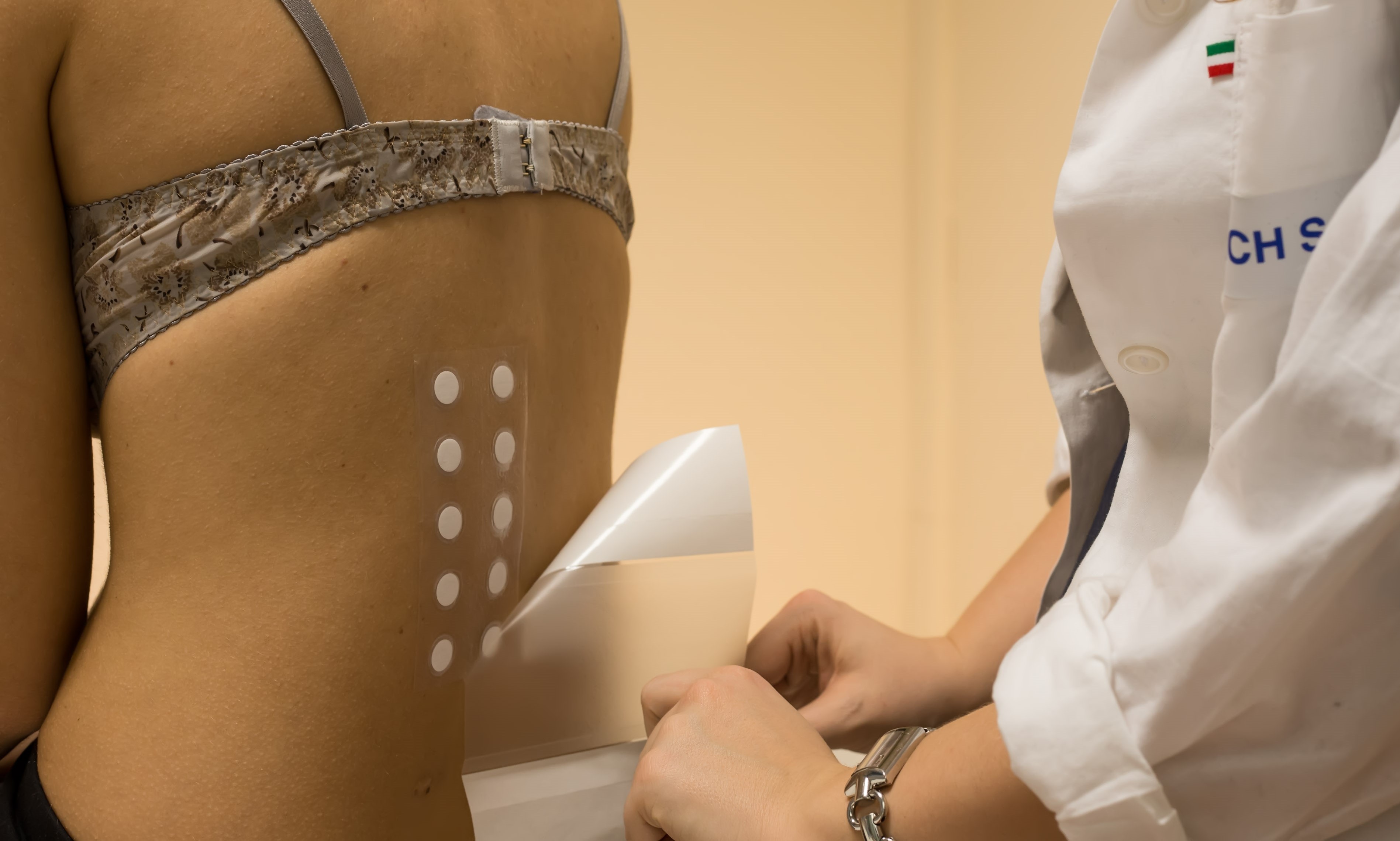
The Clinical Unit of Abich Laboratories performs tolerability and safety testing on healthy volunteers under the supervision of dermatologists, allergologists, and other medical specialists.
In-vitro safety testing offered by Abich:
- Cytotoxicity testing;
- Cytotoxicity testing: for medical devices, in line with the UNI EN ISO 10993-5 standard;
- Eye irritation testing: on monolayer cells and 3D reconstructed corneal epithelium;
- Eye corrosion testing: “fluorescein leakage” (OECD 460);
- Skin irritation testing: on cell monolayers and in-vitro reconstructed epithelia of different origin (OECD 439);
- Skin corrosion testing: (OECD 431, 435);
- Percutaneous absorption testing: (OECD 428);
- Skin sensitization testing: (THP-1, dendritic cells);
- Phototoxicity testing: (OECD 432);
- Ames testing: mutagenesis (OECD 471);
- In-vitro carcinogenesis testing: (Balb/3T3 – B.21 method, Annex V, Directive 67/548/EEC);
- Ecotoxicity testing: on Daphnia Magna (OECD 202) and seaweeds (OECD 201).
Clinical safety testing offered by Abich:
- Patch testing: repeat insult patch test and on sensitive skins;
- HIRPT for allergenic risk assessment: Human Repeat Insult Patch Test;
- Tests to evaluate the tolerability of cosmetic products: dermatological, ophthalmological, and gynaecological testing;
- Tests on sun protection products: SPF and water resistance in line with the European Cosmetics/COLIPA 2006/ISO 24444:2010 standard, in line with the FDA vol 76 n° 117 of 17 June 2011, SPF UVA PPD in line with the ISO 24442 standard, long lasting SPF test, SPF test in line with the Australian AS/NZS 2604:2012 method;
- SPF: on wet skin, to test resistance to water, sand, and sweat, persistence evaluation;
- Photopatch testing.