2002

Dr. Stefano Todeschi, CEO at Abich, and Dr. Elena Bocchietto, Director of the Abich Assay Facility, are among the founders of Abich, which was set up in Verbania, Italy, within the technological incubator of the technology park of Lake Maggiore.
2003

Abich, in collaboration with the Laboratory of Molecular and Cellular Immunology at the DIBIT of the San Raffalele Hospital in Milan, created ALLTOX, a spin-off company which has been developing tests for skin allergies to finished products, cosmetic raw ingredients, medical devices, and consumer products since 2003.
2004

Abich is awarded with the National Prize of the Italian Union of Chambers of Commerce (Unioncamere) for ‘New Innovative Enterprises’ for introducing in-vitro tests as an alternative to the use of animals, in line with OECD’s guidelines.
2005

Abich is certified to the ISO 9001 standard for the research, development, and implementation of tests and methods of chemical, physical, and microbiological analysis and on cell cultures.
2007

Abich builds a new laboratory of 1,000 square meters in Verbania, powered by solar energy.
2008
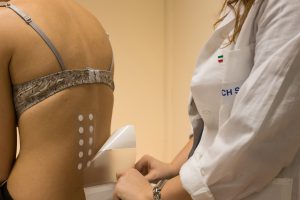
The clinical testing division of Abich laboratories is created in Vimodrone, Italy. The unit is dedicated to tolerability and efficacy testing on healthy volunteers for cosmetics for topical use on skin, skin annexes, and external mucosae.
2012
 The Abich’s Assay Facility has been certified to the GLP (Good Laboratory Practices) standard by the Ministry of Health. This certificate defines the principles by which laboratory research is programmed, performed, supervised, recorded, and reported, in order to obtain high quality experimental data.
The Abich’s Assay Facility has been certified to the GLP (Good Laboratory Practices) standard by the Ministry of Health. This certificate defines the principles by which laboratory research is programmed, performed, supervised, recorded, and reported, in order to obtain high quality experimental data.
Abich is the only Italian partner in the SMARTNANO European project on the risk posed by nanoparticles in cosmetics. The project was completed with satisfying scientific results in 2016.
2014

Abich is included in the European Community EU-NETVAL network for the validation of in-vitro alternative methods to animal testing.

Abich widens its scope by setting up Abich Inc. in Montreal, Canada, registered with the FDA (US Food and Drug Administration) for SPF (Sun Protection Factor) testing.
2016
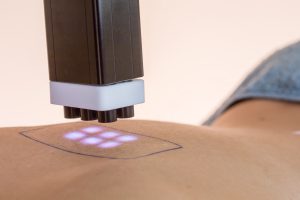
Abich builds an additional clinical division facility for cosmetic testing on healthy volunteers in Vimodrone.
2017
 Abich is certified to the ISO 9001 standard for research, development, and implementation of tests and methods of chemical, physical, and microbiological analysis and on cell cultures, for technical and formulation research and development on projects with cosmetic products, and for the assessment of the efficacy and safety of cosmetic products on human volunteers.
Abich is certified to the ISO 9001 standard for research, development, and implementation of tests and methods of chemical, physical, and microbiological analysis and on cell cultures, for technical and formulation research and development on projects with cosmetic products, and for the assessment of the efficacy and safety of cosmetic products on human volunteers.
Abich obtains the approval of the Italian Piedmont Regional Administration for the Epatocare project, which aims at identifying early biomarkers of liver fibrosis for a non-invasive diagnosis. Abich, in collaboration with the Molecular Biotechnology Centre of the University of Turin, will study extracellular microvesicles (EVs) originating in the liver and present in blood, as a possible source of diagnostic markers.
2020
 Abich obtained the UNI CEI EN ISO 13485:2016 certificate granted by the TÜV Rheinland Italia S.r.l. because the quality management system fulfils the requirements of the standards for the following scope: “Laboratory analysis and technical consultancy services for medical devices”.
Abich obtained the UNI CEI EN ISO 13485:2016 certificate granted by the TÜV Rheinland Italia S.r.l. because the quality management system fulfils the requirements of the standards for the following scope: “Laboratory analysis and technical consultancy services for medical devices”.2022

Abich joins the Lifeanalytics group enhancing and differentiating its services.