Abich a company of Lifeanalytics
The same excellence, a renewed future!

Same Expertise, New Opportunities
The trust and satisfaction of our clients have always been at the core of our work. Today, with Abich joining the Lifeanalytics group, we embark on a new chapter enriched with additional services and innovative solutions. We will continue to deliver professionalism and excellence, strengthening our commitment to offering even more.
With the support of Lifeanalytics, we aim to become your ideal partner for analysis, consultancy, training, and regulatory solutions. Thanks to Lifeanalytics’ extensive network of laboratories and locations, we provide fast and efficient support both locally and nationally. Our Competence Centers, Aid Centers, and Sampling Centers are designed to meet your needs with precision and high-quality standards.
> Click to learn more
The magical molecule of hyaluronic acid

However, you can always test to see if they can be reduced. Or not!?
We offer title checks, osmolality, viscosity, anti-age activity tests, anti-wrinkle, plumping, Botox-like effect, elasticity and toning, moisturizing effect.
The magical molecule of hyaluronic acid
The magical properties of this molecule are undeniable. It is used in our daily skincare routine, in gels, creams, serums, fillers, gauzes, even in supplements, and let’s not forget nasal and eye solutions, etc.
Thanks to its ability to retain water, it keeps the skin soft, hydrated, and visibly healthy.
At Abich – Lifeanalytics, in our chemistry department, we have been testing creams, gels, serums, fillers, and raw materials containing HA for years. We verify the concentrations of both linear and crosslinked HA using HPLC with dedicated columns and instruments, colorimetric analysis, and Elisa Kits. Contact us for tests such as solution osmolality, viscosity, stability, and compatibility with packaging. We also offer in vitro and in vivo safety and efficacy tests on your products.
Abich – Lifeanalytics is happy to assist you , email us at
Do you still have Medical Devices under the old Directive?

The Regulatory Affairs and Consulting Department of Abich-Lifeanalytics can assist and support you in the various stages of certification of your medical device according to Regulation (EU) 2017/745. On March 20, 2023, Regulation (EU) 2023/607 was published in the Official Journal of the European Union, which under certain conditions extends the validity period of certificates issued under the Directives until December 31, 2028, but despite the extension, it is important not to be caught unprepared and to meet the new obligations envisaged by the MDR correctly, starting in good time to submit the formal request to the Notified Body and, above all, to respond to their initial requests.
Abich can support you for substance-based medical devices also with the drafting of:
✅ Gap Analysis, to define all the steps to be taken to comply with MDR regulations;
✅ Qualification rationales, to define the qualification of the product as a medical device and the classification according to the new rules;
✅ Rationales supporting the mechanism of action of the medical device;
✅ Biological evaluation (BEP/BER) according to ISO 10993-1;
✅ Rationales for defining markers to be analyzed in percutaneous absorption tests;
✅ Bibliographic evaluation of the ADME of medical device ingredients.
As an integral part of the evaluation of a medical device, biocompatibility tests are an undeniable priority to assess its safety. Abich is a GLP certified laboratory that provides in vitro and in vivo biocompatibility tests aimed at the three endpoints that all medical devices are required to evaluate:
✅ Cytotoxicity: ISO 10993-5;
✅ Irritation: ISO 10993-23;
✅ Skin sensitization: ISO 10993-10.
? For more information, to receive a personalized offer, and to learn about all our services, do not hesitate to contact us at
Genotoxicity: Focus On Ames Test

If you work in R&D or in the regulatory field, you may have come across the need to assess the safety of your products or ingredients in terms of genotoxicity.
Genotoxicity testing is the scientific basis for risk assessment in different application fields including cosmetics, pharmaceutical industry (substances for pharmaceutical use and impurities, medical devices), the food sector (additives and packaging), biocidal products and many others.
International regulatory agencies have proposed various recommendations on testing strategies, and most recommend the use of a combination of tests, both in vitro and in vivo. Tests conducted for submission to regulatory agencies are generally required to be conducted in compliance to the principles of Good Laboratory Practice (GLP).
Among in vitro tests, the Ames test is the most widely used assay and is recommended by various regulatory agencies. The test uses bacteria to identify mutagenic compounds and has been shown to detect relevant genetic changes produced by the majority of genotoxic carcinogens detected by rodent assays.
It is also particularly advantageous due to the possibility to quickly provide results and to the relatively low cost compared to other tests. Moreover, it has been proved to be efficient in detecting a huge number of mutagens.
Abich-Lifeanalytics performs the Ames test according to OECD method n. 471 and is certified by the Ministry of Health to perform tests in accordance with the principles of Good Laboratory Practice (GLP).
PRINCIPLE OF THE TEST
Mutant strains of the bacterium Salmonella typhimurium are exposed to the test substance. The strains cover different types of mutations and all of them are characterized by being auxotrophic for one amino acid. In other words, all strains are unable to grow in minimal medium in the absence of histidine (tryptophan if Escherichia coli is used in place of one specific strain). In this system, the mutagenic potential of the substance is measured as a reversion rate from the auxotrophic (histidine-dependent) to the prototrophic (histidine-independent) phenotype.
Exposure to the substance is carried out both in the presence and in the absence of a rodent liver metabolic activation system in order to detect also pro-mutagenic compounds. Mutagenic potential is measured as an increase in revertant colonies capable of growing in the absence of the amino acid in the minimal culture medium.
Appropriate positive and negative controls are also included in the test. Statistical analysis of the results allows to highlight significant increases in the rate of spontaneous reversion and to detect any dose-related effect. Increase in the number of revertant colonies in just one of the experimental conditions tested indicates a mutagenic potential of the test substance. It is also possible to introduce variations to the main standard protocol, e.g. for screening compounds in R&D.
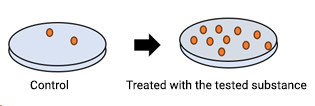
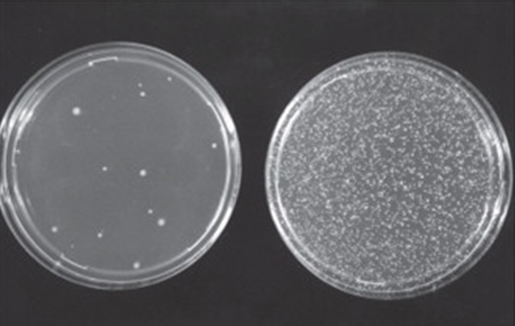
Immagine fotografica tratta da W. Föllmann et a.l in Brenner’s Encyclopedia of Genetics (Second Edition), 2013
✅ Are you developing a new product or an innovative raw material and you have to deal with the assessment of its mutagenic potential for safety purposes?
✅ We are ready to advise you on the best approach for your product and we will provide you with all the information you need to test your substance for safety!
? For inquiries, email us at
Exciting News in the Medical Field
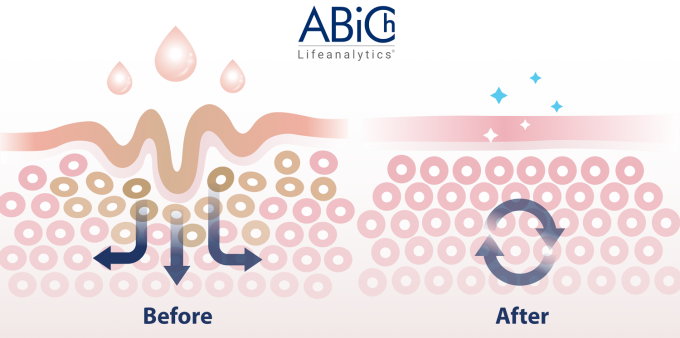
This month, let’s delve into the intriguing world of PERCUTANEOUS ABSORPTION! ??
To absorb or not to absorb? That’s the question…
With the introduction of numerous new requirements by the MDR, medical devices must now conform to higher safety standards, ensuring enhanced protection for end-users. Despite the extension granted by EU Regulation 2023/607, it’s crucial not to be caught off guard and to meet the new MDR obligations correctly. This involves timely submission of formal requests to the Notified Body and prompt responses to their initial inquiries.
At Abich, our Regulatory Affairs and Consulting department stands ready to guide you through this process, offering assistance at every stage of recertification. Particularly for substance-based medical devices, we provide invaluable support:
✅ Rationalization for defining markers in percutaneous absorption tests.
✅ Bibliographic evaluation of ADME for medical device ingredients.
✅ Absorption tests using Franz cells.
? Connect with us to delve deeper into the world of effective cosmetics! For inquiries, email us at
Don’t miss out on staying ahead in the ever-evolving medical and cosmetic industries! Reach out to Abich today for expert guidance and innovative solutions. ??
Unlocking the Secrets of Beauty: A Guide to Cosmetic Effectiveness

In the dynamic world of modern makeup, evaluating the effectiveness of products has become an essential endeavor. ? Our multifaceted methodologies, ranging from instrumental assessments to clinical and subjective evaluations, accompanied by compelling digital imagery, empower us to scrutinize and enhance cosmetic efficacy.
?️ A perennial makeup trend that transcends seasons is accentuating the eyes! With a plethora of products in the market, one standout is ???????, celebrated for its diverse claims and innovative formulations. From defined and separated lashes to waterproof, curling, and smudge-proof effects – we offer a myriad of claims backed by comprehensive testing.
? Leveraging cutting-edge image analysis tools and sophisticated software in our laboratory, we delve into the in-situ behavior of cosmetics. By monitoring and evaluating lashes before and after application, our high-resolution photos under standardized lighting conditions allow us to assess color, intensity, and retention through the analytical color follow-up technique.
? Our specially designed tests scrutinize water and humidity resistance, ensuring that waterproof formulations deliver long-lasting, impeccable results even in contact with water, tears, or sweat. Because a perfect gaze goes beyond mascara, our eyeshadow, pencil, and kajal adhere to the same principles of long-lasting wear, water and sweat resistance, and smudge-free application, always aiming for a lasting formulation.
?️? Products touching the eye area are meticulously thought out and formulated for one of the body’s most sensitive zones. Rest assured, we prioritize safety through in vivo safety testing under ophthalmological control.
? Connect with us to delve deeper into the world of effective cosmetics! For inquiries, email us at
Abich and Ecol Studio – Lifeanalytics group receive the Silver partner award at the IFSCC in London
Thank you for visiting our booth 24!

Scientific Article EPATOCARE Project
The article “Circulating Extracellular Vesicles Contain Liver-Derived RNA Species as Indicators of Severe Cholestasis-Induced Early Liver Fibrosis in Mice”, written by the researchers of Abich Srl in collaboration with the University of Turin inside the Epatocare project, has been published in the journal Antioxid Redox Signal.
Read More
Abich obtained ACCREDIA certification
The ACCREDIA Certification, issued by the Italian Accreditation body ACCREDIA, certifies that the Abich Laboratory complies with the requirements of the UNI CEI EN ISO / IEC 17025: 2018 standard for tests on surgical masks. Accredited tests are Bacterial Filtration Efficiency (BFE) and Microbial Cleanliness (Bioburden).
Read More
Abich laboratories are a part of Lifeanalytics Group
Since November 2021, Abich Srl laboratories are a part of Lifeanalytics Srl, the largest Italian analytical testing network, a consortium that includes 46 laboratories in 15 regions, sporting over 40 years of experience in analytical chemistry and microbiology services for the food, environment, pharmaceutical and cosmetics sectors.
Read More