If you work in R&D or in the regulatory field, you may have come across the need to assess the safety of your products or ingredients in terms of genotoxicity.
Genotoxicity testing is the scientific basis for risk assessment in different application fields including cosmetics, pharmaceutical industry (substances for pharmaceutical use and impurities, medical devices), the food sector (additives and packaging), biocidal products and many others.
International regulatory agencies have proposed various recommendations on testing strategies, and most recommend the use of a combination of tests, both in vitro and in vivo. Tests conducted for submission to regulatory agencies are generally required to be conducted in compliance to the principles of Good Laboratory Practice (GLP).
Among in vitro tests, the Ames test is the most widely used assay and is recommended by various regulatory agencies. The test uses bacteria to identify mutagenic compounds and has been shown to detect relevant genetic changes produced by the majority of genotoxic carcinogens detected by rodent assays.
It is also particularly advantageous due to the possibility to quickly provide results and to the relatively low cost compared to other tests. Moreover, it has been proved to be efficient in detecting a huge number of mutagens.
Abich-Lifeanalytics performs the Ames test according to OECD method n. 471 and is certified by the Ministry of Health to perform tests in accordance with the principles of Good Laboratory Practice (GLP).
PRINCIPLE OF THE TEST
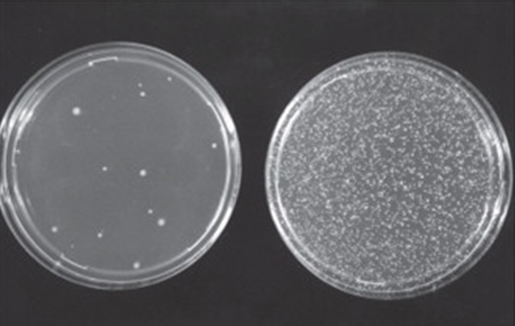
Mutant strains of the bacterium Salmonella typhimurium are exposed to the test substance. The strains cover different types of mutations and all of them are characterized by being auxotrophic for one amino acid. In other words, all strains are unable to grow in minimal medium in the absence of histidine (tryptophan if Escherichia coli is used in place of one specific strain). In this system, the mutagenic potential of the substance is measured as a reversion rate from the auxotrophic (histidine-dependent) to the prototrophic (histidine-independent) phenotype.
Exposure to the substance is carried out both in the presence and in the absence of a rodent liver metabolic activation system in order to detect also pro-mutagenic compounds. Mutagenic potential is measured as an increase in revertant colonies capable of growing in the absence of the amino acid in the minimal culture medium.
Appropriate positive and negative controls are also included in the test. Statistical analysis of the results allows to highlight significant increases in the rate of spontaneous reversion and to detect any dose-related effect. Increase in the number of revertant colonies in just one of the experimental conditions tested indicates a mutagenic potential of the test substance. It is also possible to introduce variations to the main standard protocol, e.g. for screening compounds in R&D.
Immagine fotografica tratta da W. Föllmann et a.l in Brenner’s Encyclopedia of Genetics (Second Edition), 2013
✅ Are you developing a new product or an innovative raw material and you have to deal with the assessment of its mutagenic potential for safety purposes?
✅ We are ready to advise you on the best approach for your product and we will provide you with all the information you need to test your substance for safety!
? For inquiries, email us at