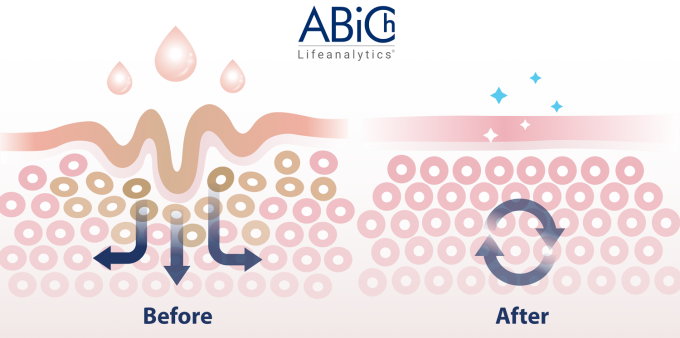
This month, let’s delve into the intriguing world of PERCUTANEOUS ABSORPTION! ??
To absorb or not to absorb? That’s the question…
With the introduction of numerous new requirements by the MDR, medical devices must now conform to higher safety standards, ensuring enhanced protection for end-users. Despite the extension granted by EU Regulation 2023/607, it’s crucial not to be caught off guard and to meet the new MDR obligations correctly. This involves timely submission of formal requests to the Notified Body and prompt responses to their initial inquiries.
At Abich, our Regulatory Affairs and Consulting department stands ready to guide you through this process, offering assistance at every stage of recertification. Particularly for substance-based medical devices, we provide invaluable support:
✅ Rationalization for defining markers in percutaneous absorption tests.
✅ Bibliographic evaluation of ADME for medical device ingredients.
✅ Absorption tests using Franz cells.
? Connect with us to delve deeper into the world of effective cosmetics! For inquiries, email us at
Don’t miss out on staying ahead in the ever-evolving medical and cosmetic industries! Reach out to Abich today for expert guidance and innovative solutions. ??