Test for the determination of UVA photoprotection in vivo
Anti-wrinkle activity through skin profilometry
| Regulatory reference: | Proprietary method |
| Claim: | Anti-aging, Anti-wrinkle, Reduces signs of aging |
| Application field: | Raw materials and finished products. |
| Description of the test: | Blue laser skin profilometry: this technique allows you to carry out cosmetological studies of anti-wrinkle products with great reproducibility and accuracy thanks to a sophisticated instrumental and electronic set-up, which allows you to normalize the position of the volunteer and the test area in a millimetric way, an indispensable condition in conducting an anti-aging clinical study for the reduction of facial wrinkles. This clinical study allows a quantitative experimental evaluation of the anti-wrinkle effect through 2D measurements of wrinkles (Ra, Rz, LR, SPa, SPt, SPTm), and 3D (volume, average and maximum depth of wrinkles), and through quantitative morphological evaluations (mm2 of total surface covered by wrinkles). This allows the anti-wrinkle effect of any product to be assessed in the most appropriate way: immediate tensor effect, filler, firming effects (firming, toning, plumping, etc.) |
| Photo: | 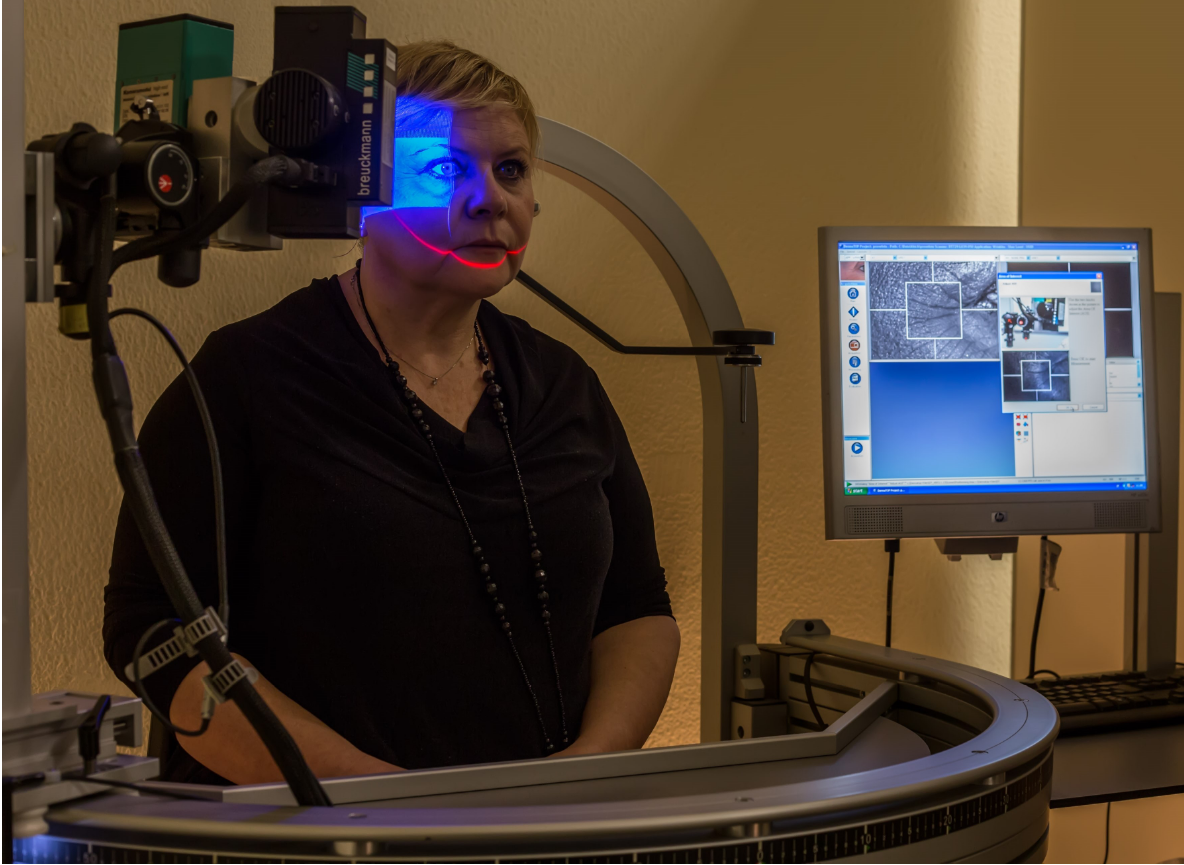 |
H295R Steroidogenesis Assay – OECD 456
| Regulatory reference: | According to OECD 456. |
| Claim: | No endocrine disruption |
| Application field: | Raw ingredients and finished products. |
| Description of the test: | The purpose of the test is identifying substances that interfere with 17-ß estradiol and testosterone production. |
Test of reduction of SPF caused by salt water
| Regulatory reference: | ISO 24444:2010 of 15/11/2010 and Colipa test method 2006 for the determination of the WATER RESISTANCE. Test performed according to modified COLIPA 2005 method |
| Claim: | Salt water resistant |
| Application field: | Finished sunscreen products. |
| Description of the test: | Test performed on 10 volunteers, by comparison of pre-wash and post-wash SPF. The treatment includes two washes, one in salt water, performed by irroration with 1M NaCl solution by a pressureless drop shower, and one in fresh water. If the reduction of protection is greater than 50% the product isn’t considered to be “Salt Water Resistant”. |
Hyaluronic acid synthesis test
| Regulatory reference: | Proprietary method. |
| Claim: | Anti-aging and Reduces signs of aging |
| Application field: | Raw materials and finished products. |
| Description of the test: | In vitro evaluation of the synthesis of hyaluronic acid up to 48h following exposure to the sample, in several concentrations, on skin and dermal papillae fibroblasts. |
Test of reduction of SPF caused by sand
| Regulatory reference: | ISO 24444:2010 of 15/11/2010 and Colipa test method 2006 for SPF. |
| Claim: | Sand resistant |
| Application field: | Finished products. |
| Description of the test: | In vivo test on 10 subjects. Comparison between SPF of sun product and the reduction on protection cause of double sand static contact of 20 minutes in this sequence: contact, techanical cleaning, contact and final mechanical cleaning before the exposure to the solar multiport simulator. If the reduction of protection is mayor than 50% the product isn’t “sand resistant”. |
In vitro test for the evaluation of the protective effect against air pollution
| Regulatory reference: | Proprietary method |
| Claim: | Anti-pollution and Antioxidant |
| Application field: | Raw materials and finished products. |
| Description of the test: | In vitro test for the evaluation of the protective effect against air pollution. The purpose of this test is to evaluate the efficacy of the sample in reducing ROS release in response to a mix of heavy metals and atmospheric dust particulate in a skin-derived cell model. |
Cytotoxicity test for the identification of substances with acute oral toxicity LD50>2000 mg/kg
| Regulatory reference: | OECD 129: on using cytotoxicity tests to estimate starting doses for acute oral systemic toxicity tests; EURL-ECVAM 2011: follow-up study on the predictive capacity of the 3T3 Neutral Red Uptake cytotoxicity assay to correctly identify substances not classified for acute oral toxicity under the EU CLP system. |
| Claim: | Biocompatible and Non citotoxic |
| Application field: | Chemical substances or mixtures. |
| Description of the test: | The in vitro LD50 prediction test is designed to test chemicals or mixtures by evaluating their cytotoxic effect on an in vitro model of BALB/c 3T3 murine fibroblasts. |
Evaluation of the enzymatic inhibition of Tyrosinase
| Regulatory reference: | Proprietary method |
| Claim: | Depigmenting action |
| Application field: | Active ingredients, mixtures or finished products. |
| Description of the test: | Evaluation of the efficacy of a sample in inhibiting the activity of the tyrosinase enzyme in a controlled in vitro direct reaction. |
Evaluation of proliferation and protein synthesis on dermal papilla mesenchymal cells
| Regulatory reference: | Proprietary method |
| Claim: | Hair loss protection and Strenghtener |
| Application field: | Active ingredients and finished products. |
| Description of the test: | Quantitative evaluation of the effects of a finished product or active ingredient on cell proliferation and protein synthesis through MTT test and total protein quantification after different periods of exposure. |